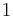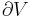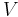Thermodynamic equilibrium
| Thermodynamics | |||||||||||||||||||||||||||||||||||||||||||||||

|
|||||||||||||||||||||||||||||||||||||||||||||||
| Concepts in Chemical Equilibria |
|---|
| Acid dissociation constant |
| Binding constant |
| Binding selectivity |
| Buffer solution |
| Chemical equilibrium |
| Chemical stability |
| Dissociation constant |
| Distribution coefficient |
| Distribution ratio |
| Dynamic equilibrium |
| Equilibrium chemistry |
| Equilibrium constant |
| Equilibrium unfolding |
| Equilibrium stage |
| Liquid-liquid extraction |
| Phase diagram |
| Predominance diagram |
| Phase rule |
| Reaction quotient |
| Solubility equilibrium |
| Stability constants of complexes |
| Thermodynamic equilibrium |
| Vapor-liquid equilibrium |
| edit |
In thermodynamics, a thermodynamic system is said to be in thermodynamic equilibrium when it is in thermal equilibrium, mechanical equilibrium, radiative equilibrium, and chemical equilibrium. The word equilibrium means a state of balance. In an equilibrium state, there are no unbalanced potentials (or driving forces) with the system. A system that is in equilibrium experiences no changes when it is isolated from its surroundings.
The opposite of equilibrium systems are nonequilibrium systems that are instantaneously off balance.
Contents |
Overview
Classical thermodynamics deals with dynamic equilibrium states. The local state of a system at thermodynamic equilibrium is determined by the values of its intensive parameters, such as pressure or temperature. Specifically, thermodynamic equilibrium is characterized by the minimum of a thermodynamic potential, such as the Helmholtz free energy, i.e. systems at constant temperature and volume:
-
- A = U – TS;
Or as the Gibbs free energy, i.e. systems at constant pressure and temperature:
-
- G = H – TS.
where T = temperature, S = entropy, p = pressure, V = volume. The Helmholtz free energy is often denoted by the symbol F, but the use of A is preferred by IUPAC [2].
The process that leads to a thermodynamic equilibrium is called thermalization. An example of this is a system of interacting particles that is left undisturbed by outside influences. By interacting, they will share energy/momentum among themselves and reach a state where the global statistics are unchanging in time.
Conditions for equilibrium
By considering the differential form of thermodynamic potentials, the following relationships can be derived:
- For a completely isolated system, ΔS = 0 at equilibrium.
- For a system at constant temperature and volume, ΔA = 0 at equilibrium.
- For a system at constant temperature and pressure, ΔG = 0 at equilibrium.
The various types of equilibriums are achieved as follows:
- Two systems are in thermal equilibrium when their temperatures are the same.
- Two systems are in mechanical equilibrium when their pressures are the same.
- Two systems are in diffusive equilibrium when their chemical potentials are the same.
Local and global equilibrium
It is useful to distinguish between global and local thermodynamic equilibrium. In thermodynamics, exchanges within a system and between the system and the outside are controlled by intensive parameters. As an example, temperature controls heat exchanges. Global thermodynamic equilibrium (GTE) means that those intensive parameters are homogeneous throughout the whole system, while local thermodynamic equilibrium (LTE) means that those intensive parameters are varying in space and time, but are varying so slowly that for any point, one can assume thermodynamic equilibrium in some neighborhood about that point.
If the description of the system requires variations in the intensive parameters that are too large, the very assumptions upon which the definitions of these intensive parameters are based will break down, and the system will be in neither global nor local equilibrium. For example, it takes a certain number of collisions for a particle to equilibrate to its surroundings. If the average distance it has moved during these collisions removes it from the neighborhood it is equilibrating to, it will never equilibrate, and there will be no LTE. Temperature is, by definition, proportional to the average internal energy of an equilibrated neighborhood. Since there is no equilibrated neighborhood, the concept of temperature breaks down, and the temperature becomes undefined.
It is important to note that this local equilibrium applies only to massive particles. In a radiating gas, the photons being emitted and absorbed by the gas need not be in thermodynamic equilibrium with each other or with the massive particles of the gas in order for LTE to exist.
As an example, LTE will exist in a glass of water which contains a melting ice cube. The temperature inside the glass can be defined at any point, but it is colder near the ice cube than far away from it. If energies of the molecules located near a given point are observed, they will be distributed according to the Maxwell-Boltzmann distribution for a certain temperature. If the energies of the molecules located near another point are observed, they will be distributed according to the Maxwell-Boltzmann distribution for another temperature.
Local thermodynamic equilibrium is not a stable state, unless it is maintained by exchanges between the system and the outside. For example, it could be maintained inside the glass of water by regularly adding ice into it in order to compensate for the melting. Transport phenomena are processes which lead a system from local to global thermodynamic equilibrium. Going back to our example, the diffusion of heat will lead our glass of water toward global thermodynamic equilibrium, a state in which the temperature of the glass is completely homogeneous.[1]
Types of equilibrium
Thermal equilibrium
Thermal equilibrium is achieved when two systems in thermal contact with each other cease to exchange energy by heat. If two systems are in thermal equilibrium their temperatures are the same.
Thermal equilibrium occurs when a system's macroscopic thermal observables have ceased to change with time. For example, an ideal gas whose distribution function has stabilised to a specific Maxwell-Boltzmann distribution would be in thermal equilibrium. This outcome allows a single temperature and pressure to be attributed to the whole system. Thermal equilibrium of a system does not imply absolute uniformity within a system; for example, a river system can be in thermal equilibrium when the macroscopic temperature distribution is stable and not changing in time, even though the spatial temperature distribution reflects thermal pollution inputs.
Quasistatic equilibrium
Quasistatic equilibrium is the quasi-balanced state of a thermodynamic system near to thermodynamic equilibrium, in some sense. In a quasistatic or equilibrium process, a sufficiently slow transition of a thermodynamic system from one equilibrium state to another occurs such that at every moment in time the state of the system is close to an equilibrium state. During a quasistatic process, the system reaches equilibrium much faster, almost instantaneously, than its physical parameters vary.
Non-equilibrium
Non-equilibrium thermodynamics is a branch of thermodynamics that deals with systems that are not in thermodynamic equilibrium. Most systems found in nature are not in thermodynamic equilibrium because they are not in stationary states, and are continuously and discontinuously subject to flux of matter and energy to and from other systems. The thermodynamic study of non-equilibrium systems requires more general concepts than are dealt with by equilibrium thermodynamics. Many natural systems still today remain beyond the scope of currently known macroscopic thermodynamic methods.
Planetary Equilibrium Temperature
General references
- Cesare Barbieri (2007) Fundamentals of Astronomy. First Edition (QB43.3.B37 2006) CRC Press ISBN 0-7503-0886-9, 9780750308861
- Hans R. Griem (2005) Principles of Plasma Spectroscopy (Cambridge Monographs on Plasma Physics), Cambridge University Press, New York ISBN 0-521-61941-6
- C. Michael Hogan, Leda C. Patmore and Harry Seidman (1973) Statistical Prediction of Dynamic Thermal Equilibrium Temperatures using Standard Meteorological Data Bases, Second Edition (EPA-660/2-73-003 2006) United States Environmental Protection Agency Office of Research and Development, Washington DC [1]
- F. Mandl (1988) Statistical Physics, Second Edition, John Wiley & Sons
Footnotes
External links
- Breakdown of Local Thermodynamic Equilibrium George W. Collins, The Fundamentals of Stellar Astrophysics, Chapter 15
- Thermodynamic Equilibrium, Local and otherwise lecture by Michael Richmond
- Non-Local Thermodynamic Equilibrium in Cloudy Planetary Atmospheres Paper by R. E. Samueison quantifying the effects due to non-LTE in a atmosphere
- Local Thermodynamic Equilibrium
- Xchanger Inc, webpage Calculator for thermodynamic equilibrium of pneumatic conveying air and conveyed product.